Determine Whether Each Molecule Given Below Is Polar or Nonpolar
These bonds dont form molecules that. Consider stearic acid shown below which has 18 carbons.

Oneclass Need Help To Answer These Two Questions Please Drag The Appropriate Items To Their Respecti
For example you can predict which solvents will be most effective with a given chemical if you know its.

. The molecule is nonpolar with sp2 hybridization and LDF attractions. Determine whether each of the given characteristics refers to electrolytes or non-electrolytes. In a solution a polar molecule cannot be mixed with the non-polar.
For example only a 3-fold term ie. Each F is filled with eight. Polar samples are thus retained on the polar surface of the column packing longer than less polar materials.
Arrangement of only the atoms in a molecule- molecular geometry. _____ b-because there no overall net dipole. A Lewis structure for CH₃CHCH₂ is shown below on the left.
Boron has only 6 electrons. Generally solutes are soluble in solvents that are most similar to them molecularly. Polar solutes will dissolve better in polar solvents and non-polar solutes will dissolve better in non-polar solvents.
It is often an octet exception due to its small size and having only 5 protons. This glossary of chemistry terms is a list of terms and definitions relevant to chemistry including chemical laws diagrams and formulae laboratory tools glassware and equipmentChemistry is a physical science concerned with the composition structure and properties of matter as well as the changes it undergoes during chemical reactions. Determine if the molecule is polar or nonpolar.
The weak bonds are of three types. N3 is appropriate for the H-C-C-H dihedral in ethane while a 2-fold term is typically required for the treatment of double bonds such as the H-CC-H dihedral in. Image 29 A Nonpolar Correct the ring structure is symmetrical.
For example sugar is a polar solute and absorbs very well in water. The polarity of a molecule tells whether the electron cloud is equally distributed across the atoms within the molecule or whether an electronegative atom is affecting the electron density. The distribution of the electrons will affect the behavior and reactivity of the molecule.
Here the electrons are shared almost equally. These involve atoms in the polypeptide backbone as well as atoms in the amino acid side chains. Nonpolar Explain why you chose the answer above.
Linear F2- nonpolar Trigonal planar BCL3- nonpolar Tetrahedral Sif4- nonpolar. Because CO is a polar molecule it experiences dipole-dipole attractions. We have seen that the solubility properties of molecules strongly depend on how much of the molecule is polar and how much is nonpolar.
Determine whether each of the molecules below is polar or nonpolar. It features an extensive vocabulary and a. Predict whether bonding angles A and B will be equal to greater than or less than the ideal bonding angles according to the VSEPR model.
Phet molecule polarity simulation answer key. The folding of a protein chain is however further constrained by many different sets of weak noncovalent bonds that form between one part of the chain and another. The molecular geometry is trigonal planar.
The complete molecule will be a polar molecule. Nonpolar Explain why you chose the answer above. If the difference is below about 05 the bond is nonpolar covalent.
The stationary phase is nonpolar hydrophobic in nature while the mobile phase is a polar liquid such as mixtures of water and methanol or acetonitrile. Determine whether the bonds between the following are polar polar covalent or ionic. CO2 is non-polar because of its symmetrical geometry and the dipole moment generated along with the CO bond also canceled out each other as the molecular shape of CO2 is linear and it has Sp hybridization with a bond angle of 180º which makes it a highly symmetrical molecule.
Because N 2 is nonpolar its molecules cannot exhibit dipole-dipole attractions. The end containing the two oxygens shown in red is polar but the rest of the molecule is completely nonpolar shown in blue. Other 9 pairs and place them around each terminal fluorine atom.
Draw the Lewis structure. OH Tally the valence electrons. ___a-because there a overall net dipole from the very electronegative oxygen atom.
Determine the end-to-end separation direct distance between molecule ends in Angstroms of a coiled PTFE molecule with a molecular weight of 692484 gmol. A molecule or atom which does not have any charges present at the end due to the reason that electrons are equally distributed and those which symmetrically cancel out each other are the non- polar molecules. There is an uneven distribution of electron charge on the whole molecule.
Hydrogen bonds ionic bonds and van der Waals attractions as explained in. The dipole-dipole attractions between CO molecules are comparably stronger than the dispersion forces between nonpolar N 2 molecules so CO is expected to have the higher boiling point. If a molecule does not have this ionic makeup it is considered nonpolar.
Note that typically not all the cosine terms associated with each multiplicity are suitable for an accurate description of a given torsion. If the C-C axis of the given molecule is the y axis and the x axis is up and down in the plane of the.

Is The Molecule Ch3ch2oh Polar Or Nonpolar Explain Study Com
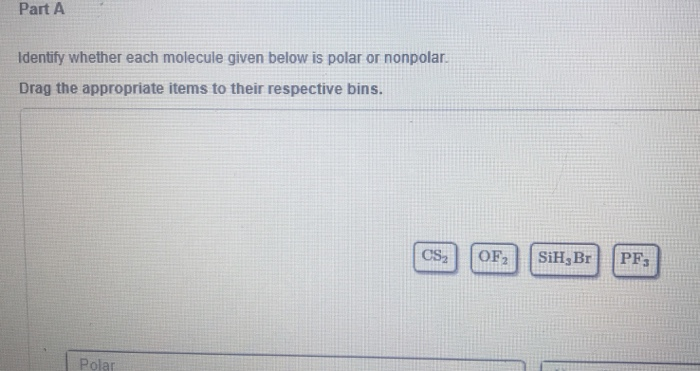
Solved Part A Identify Whether Each Molecule Given Below Is Chegg Com
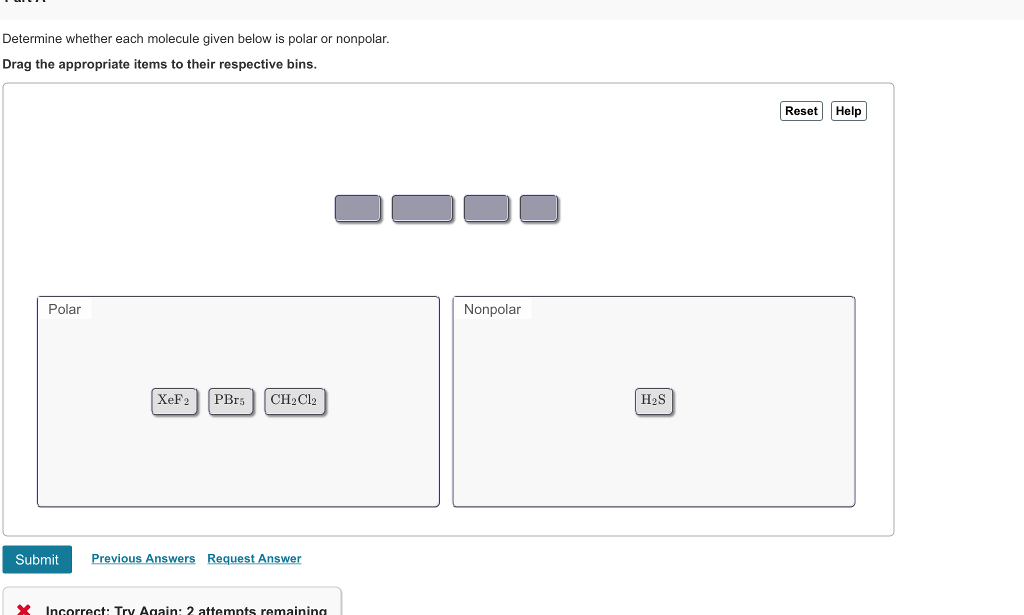
Solved Determine Whether Each Molecule Given Below Is Polar Chegg Com

Polar And Nonpolar Molecules How To Tell If A Molecule Is Polar Or Nonpolar Youtube

Oneclass Ory Bookmarks Window Help E I Session Masteringchemistr A Course Home I Session Mast

Solved Identify Whether Each Molecule Given Below Is Polar Chegg Com
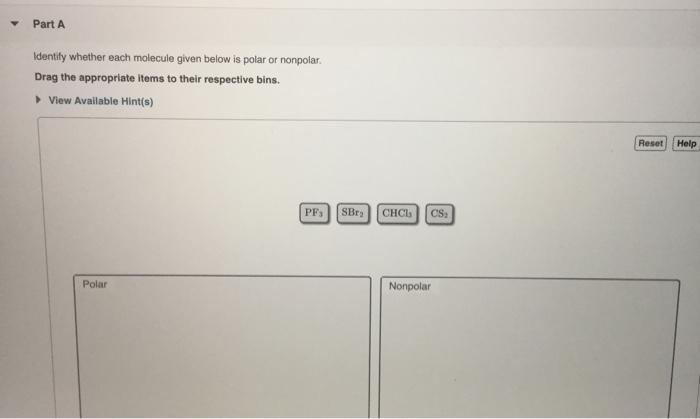
Solved Part A Identify Whether Each Molecule Given Below Is Chegg Com
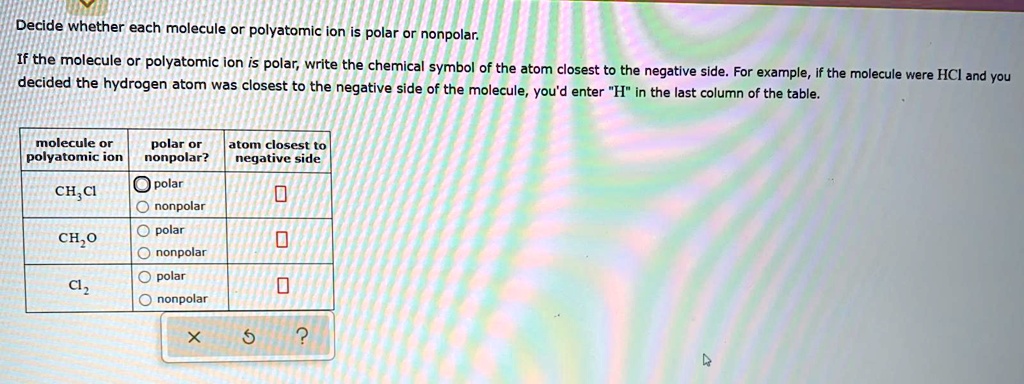
Solved Decide Whether Each Molecule Or Polyatomic Ion Is Polar Or Nonpolar If The Molecule Or Polyatomic Ion Is Polar Write The Chemical Symbol Of The Atom Closest To The Negative Side For
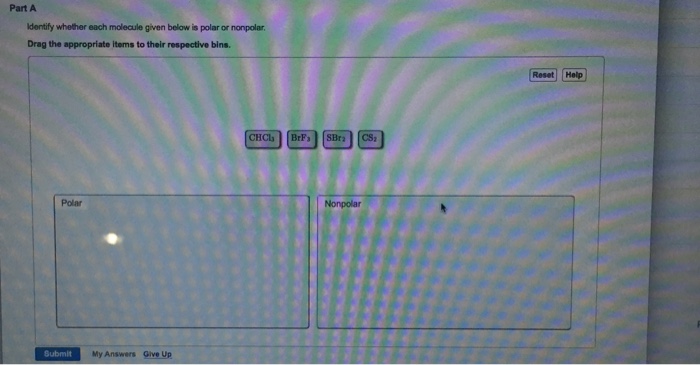
Solved Part A Dentify Whether Each Molecule Given Below Is Chegg Com
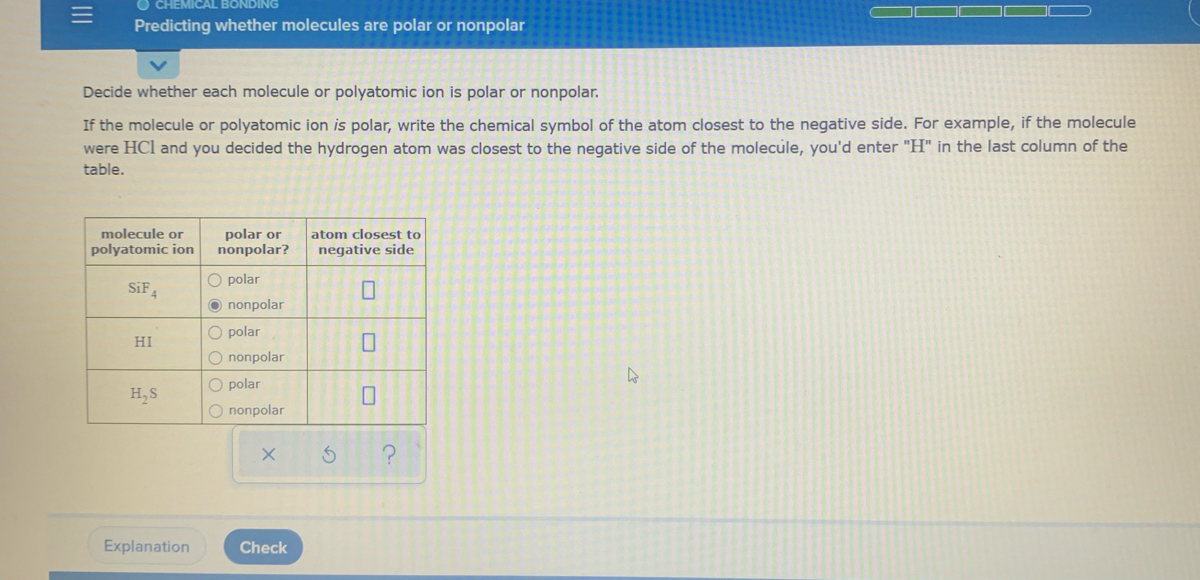
Answered Decide Whether Each Molecule Or Bartleby

Solved Indicate Whether Each Molecule Is Polar Or Nonpolar Chegg Com
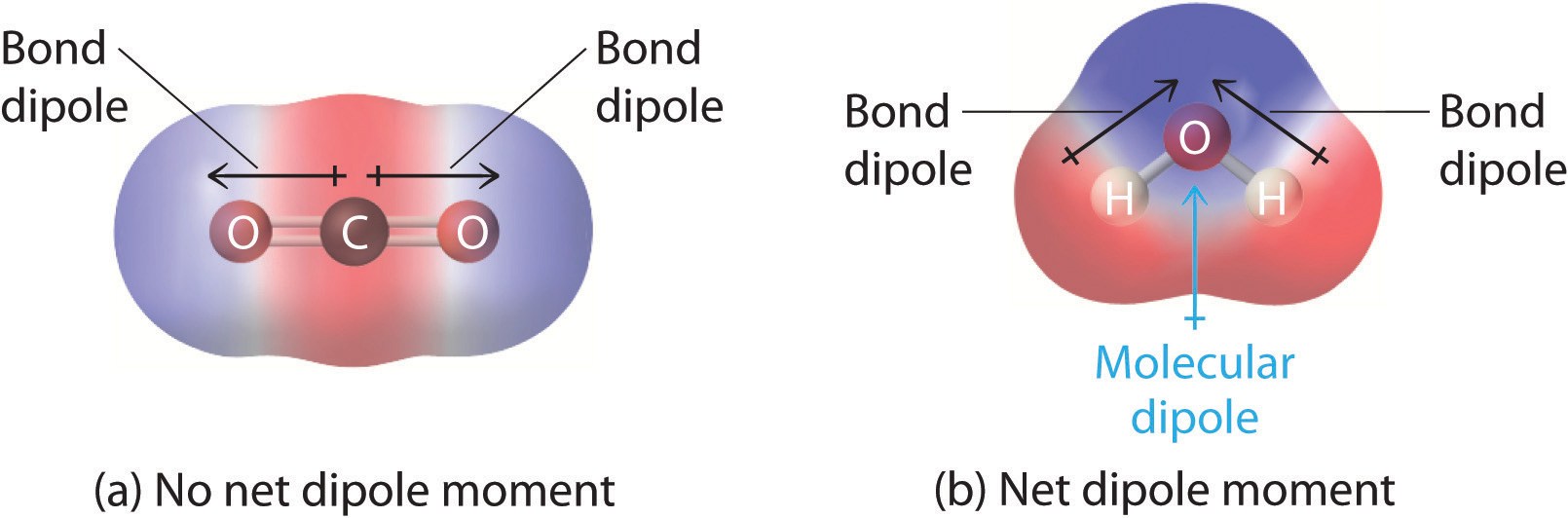
1 9 Electronegativity And Bond Polarity Review Chemistry Libretexts

Molecular Shape And Polarity How To Determine Whether A Molecule Will Be Polar Or Nonpolar Youtube

Solved Identify Whether Each Molecule Given Is Polar Or Non Chegg Com
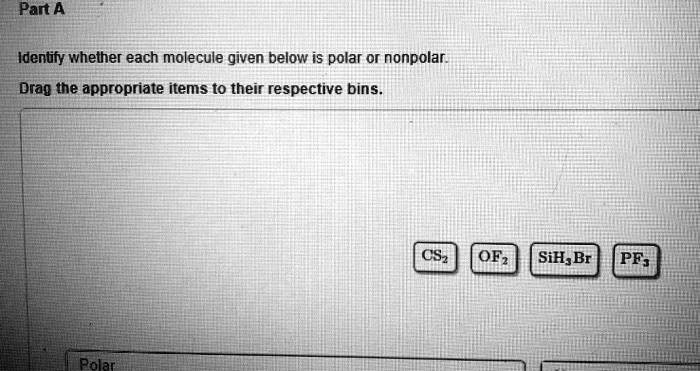
Solved Part A Identify Whether Each Molecule Given Below Is Polar Or Nonpolar Drag The Appropriate Items To Their Respective Bins Csz Ofz Sih Br Pf Pola
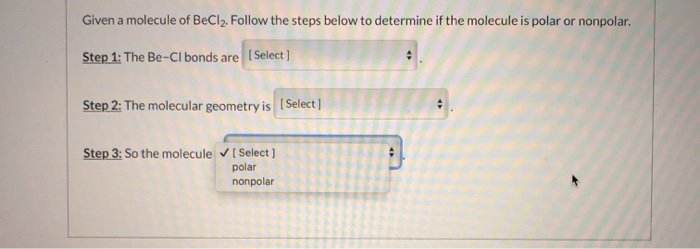
Solved Given A Molecule Of Becl2 Follow The Steps Below To Chegg Com
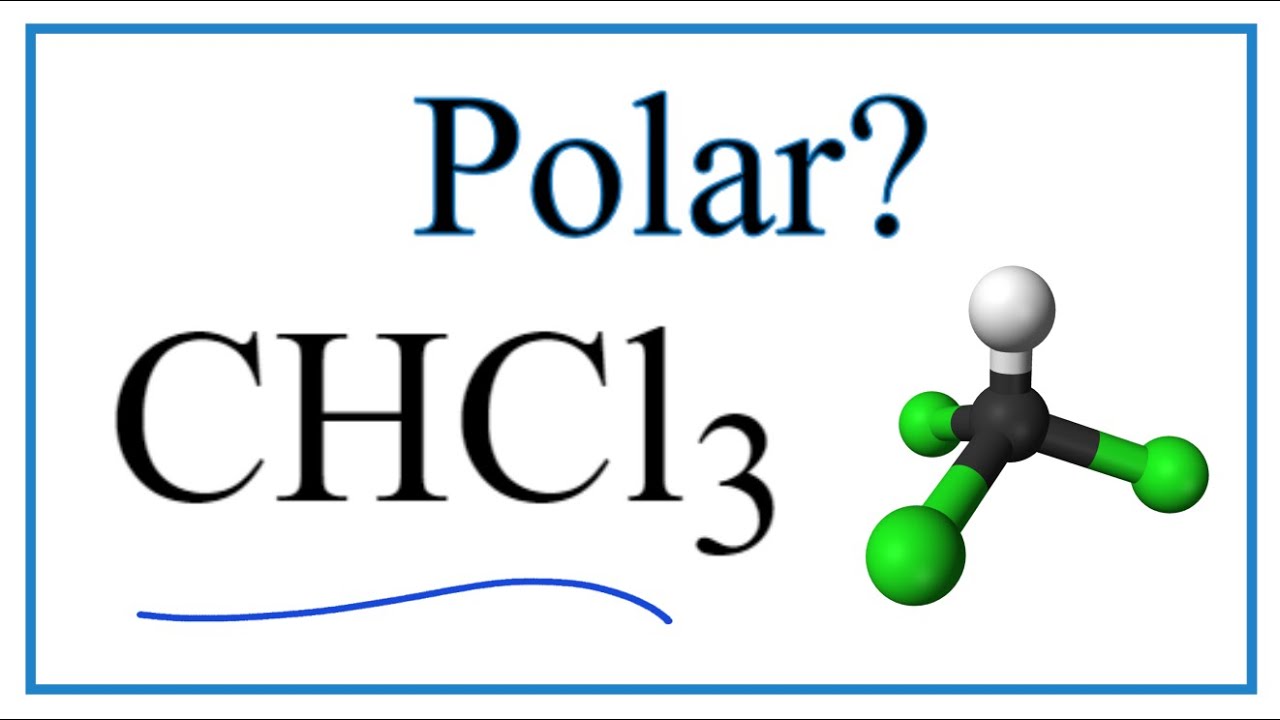
Is Chcl3 Polar Or Nonpolar Trichloromethane Or Chloroform Youtube


Comments
Post a Comment